Recording Available: Lunch and Learn – Telehealth Policy Developments COVID-19 and Beyond
The Alliance for Connected Care reviewed the multitude of legislative and regulatory changes for telehealth and remote patient monitoring over the past month and discussed new opportunities beyond COVID-19. Presentation PDF
Alliance Comments on Medicare Advantage Regulation
The Alliance for Connected Care commented on the Centers for Medicare and Medicaid Services proposed rule on “Medicare and Medicaid Programs; Contract Year 2021 and 2022 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicaid Program, Medicare Cost Plan Program, and Programs of All-Inclusive Care for the Elderly.”
In our comments, we address four key areas for telehealth and remote patient monitoring: 1) Telehealth for Medicare Advantage network adequacy, 2) telehealth for Special Needs Plans, 3) additional telehealth benefit requirements, and 4) telehealth changes to the medical loss ratio.
Please find the full comments below.
 Loading...
Loading...
Telehealth Funding Announcements and Opportunities – COVID-19
The Alliance has compiled telehealth funding opportunities below. A list of appropriations from the third COVID-19 legislative package, the CARES Act, can be viewed here.
Federal Communications Commission Report and Order
On April 2, FCC released a report and order on the COVID-19 Telehealth Program and Connected Care Pilot Program. These programs provide funding for telecommunications, and telehealth services to nonprofit healthcare organizations.
COVID-19 Telehealth Program – $200 million to be used immediately (APPLY HERE)
- The COVID-19 Telehealth Program will be open to eligible health care providers, whether located in rural or non-rural areas, and will provide eligible health care providers support to purchase telecommunications, information services, and connected devices to provide connected care services in response to the coronavirus pandemic. The COVID-19 Telehealth Program will only fund monitoring devices (e.g., pulse-ox, BP monitoring devices), that are themselves connected. The COVID-19 Telehealth Program will not fund unconnected devices that patients can use at home and then share the results with their medical professional remotely.
- The COVID-19 Telehealth Program will provide selected applicants full funding for eligible services and devices through its congressionally appropriated $200 million budget, and these funds will be available until they are expended or until the current pandemic has ended. FCC does not anticipate awarding more than $1 million to any single applicant.
- Eligibility: (1) post-secondary educational institutions offering health care instruction, teaching hospitals, and medical schools; (2) community health centers or health centers providing health care to migrants; (3) local health departments or agencies; (4) community mental health centers; (5) not-for-profit hospitals; (6) rural health clinics; (7) skilled nursing facilities; or (8) consortia of health care providers consisting of one or more entities falling into the first seven categories.
- Applications will be accepted after publication of this Report and Order and notice of OMB’s approval of the COVID-19 Telehealth Program information collection requirements in the Federal Register.
Connected Care Pilot Program – up to $100 million in total funding over three years
- The Pilot Program we adopt today is a discrete, limited duration program that will provide universal service support to help defray health care providers’ qualifying costs of providing connected care services, with a primary focus on providing these services to low-income or veteran patients.
- The Pilot Program will provide funding for selected pilot projects to cover 85% of the eligible costs of broadband connectivity, network equipment, and information services necessary to provide connected care services to the intended patient population. They decline to set a number of pilot projects or indicate an expected amount per project and will evaluate proposals received.
- They seek diverse participation to maximize the potential for the Pilot Program to provide meaningful data about the benefits of connected care, and how and whether Universal Service Fund support could be used more broadly in the future to enable the adoption of connected care services among patients and their health care providers.
- Eligibility: (1) post-secondary educational institutions offering health care instruction, teaching hospitals, and medical schools; (2) community health centers or health centers providing health care to migrants; (3) local health departments or agencies; (4) community mental health centers; (5) not-for-profit hospitals; (6) rural health clinics; (7) skilled nursing facilities; or (8) consortia of health care providers consisting of one or more entities falling into the first seven categories.
Health Resources & Services Administration Funding
- The CARES Act signed into law on March 27, provides HRSA $275 million to remain available until September 30, 2022 for Ryan White programs, rural health programs, and telehealth programs.
- On March 24, HHS, through HRSA, awarded $100 million to 1,381 health centers across the nation to expand screening and testing, acquire additional medical supplies and expand telehealth capacity in response to the COVID-19 pandemic. The funding was provided by the Coronavirus Preparedness and Response Supplemental Appropriations Act, the first coronavirus relief package.
Agency for Healthcare Research and Quality Funding Opportunity
- On March 26, AHRQ issued a notice of intent to publish a funding opportunity to support novel, high-impact studies evaluating health system and healthcare professional responsiveness to the COVID-19 pandemic. AHRQ is interested in challenges with the rapid expansion of telemedicine, and how digital health innovations have contributed to health system and provider capabilities to meet the needs of vulnerable populations. AHRQ plans to make up to $5 million available.
Department of Defense Funding Opportunity
- The Medical Technology Enterprise Consortium (MTEC) posted a pre-announcement request for project proposals (RPP) to develop and deploy the National Emergency Telecritical Care Network (NETCCN) — a cloud-based, low-resource, stand-alone health information management system to create virtual critical care wards. The U.S. Government (USG) Department of Defense (DoD) is anticipated to have approximately $30 to $37 million in FY20 funds. The upcoming RPP(s) will be posted to the MTEC website (mtec-sc.org).
Letter to CMS on MA-PD Rule
The Alliance submitted comments on the MA-PD Rule. Within the Proposed Rule, we have identified four priorities for telehealth and connected care:
- Medicare Advantage (MA) and Cost Plan Network Adequacy
- Improvements to Care Management Requirements for Special Needs Plans (SNPs)
- Additional Telehealth Benefits
- Medical Loss Ratio (MLR)
Mount Sinai Uses Remote Patient Monitoring to Rapidly Respond to COVID-19
Mount Sinai Uses Remote Patient Monitoring to Rapidly Respond to COVID-19
A new remote monitoring platform developed by the Mount Sinai Health System is helping health care providers to care for COVID-19 patients who are recovering at home. With the platform, providers can monitor the patients’ symptoms and adjust care as necessary, including sending the patient to the hospital if symptoms worsen.
A patient can enroll in the program by a Mount Sinai Health System physician or the patient can text the words “Precision Recovery” to (332) 213-9130. A Mount Sinai provider from the Precision Recovery team will then contact them as soon as possible to establish an online video chat for on-boarding. As part of the on-boarding, the patient downloads a daily symptom tracking application, MyCap, which is completed via smart device to help the team track symptoms of the virus, such as body temperature, cough, breathing levels, and body aches. If a health care provider on the team sees concerning data during the home monitoring, the provider can enlist one of the Mount Sinai doctors on the team to have an online video chat with the patient. If necessary, they can organize an emergency medical team for mobilization.
Adherence to and Retention in Medications for Opioid Use Disorder Among Adolescents and Young Adults
Adherence to and Retention in Medications for Opioid Use Disorder Among Adolescents and Young Adults
Abstract
The volatile opioid epidemic is associated with higher levels of opioid use disorder (OUD) and negative health outcomes in adolescents and young adults. Medications for opioid use disorder (MOUD) demonstrate the best evidence for treating OUD. Adherence to and retention in MOUD, defined as continuous engagement in treatment, among adolescents and young adults, however, is incompletely understood. We examined the state of the literature regarding the association of age with adherence to and retention in MOUD using methadone, buprenorphine, or naltrexone among persons aged 10–24 years, along with related facilitators and barriers. All studies of MOUD were searched for that examined adherence, retention, or related concepts as an outcome variable and included adolescents or young adults. Search criteria generated 10,229 records; after removing duplicates and screening titles and abstracts, 587 studies were identified for full-text review. Ultimately, 52 articles met inclusion criteria for abstraction and 17 were selected for qualitative coding and analysis. Younger age was consistently associated with shorter retention, although the overall quality of included studies was low. Several factors at the individual, interpersonal, and institutional levels, such as concurrent substance use, MOUD adherence, family conflict, and MOUD dosage and flexibility, appeared to have roles in MOUD retention among adolescents and young adults. Ways MOUD providers can tailor treatment to increase retention of adolescents and young adults are highlighted, as is the need for more research explaining MOUD adherence and retention disparities in this age group.
Recording Available: Virtual Hill Briefing – Using Telehealth to Address the Coronavirus Public Health Emergency
During this event, telehealth leaders spoke to what their systems are doing and how they are using new flexibility created by Congress to deliver telehealth during the COVID-19 pandemic. A couple of teaser screenshots are included below. Presentation PDF.
Dr. Ethan Booker, Medical Director at MedStar Telehealth Innovation Center
“In February, we thought we had a decent month, with about 240 telehealth video visits delivered to people’s homes. Since March 13, we have completed more than 12,000 video visits and now have approximately 1000 providers in our health care system using telehealth to provide care.”
“We used to do about 5-6 of [scheduled outpatient visits] a week, as you can see here, we did more than a 1,000 Friday and it has continued to grow through the early part of the week.”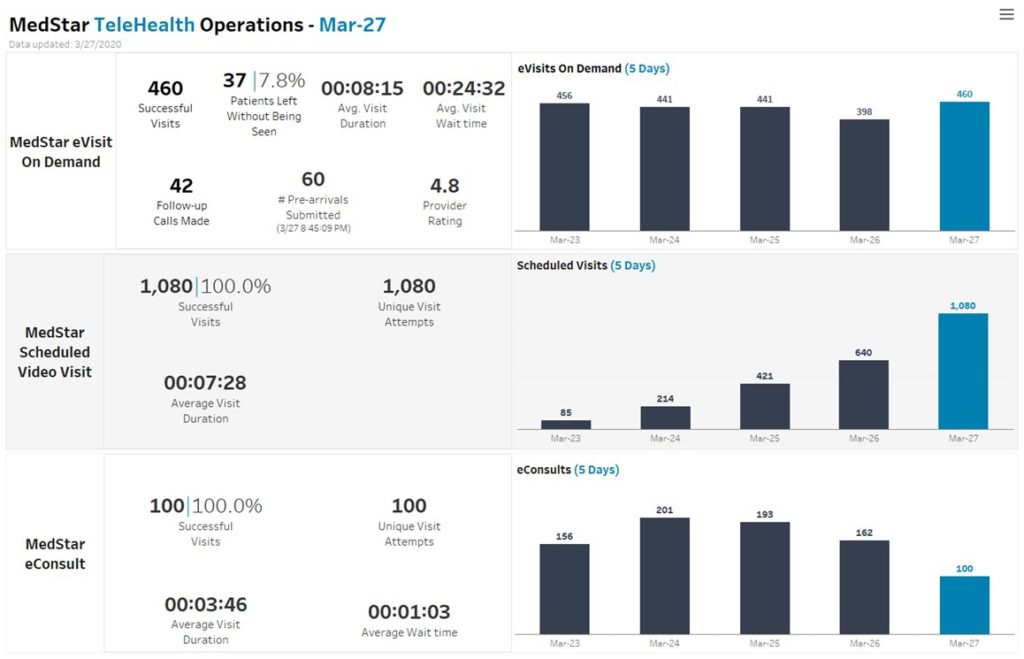
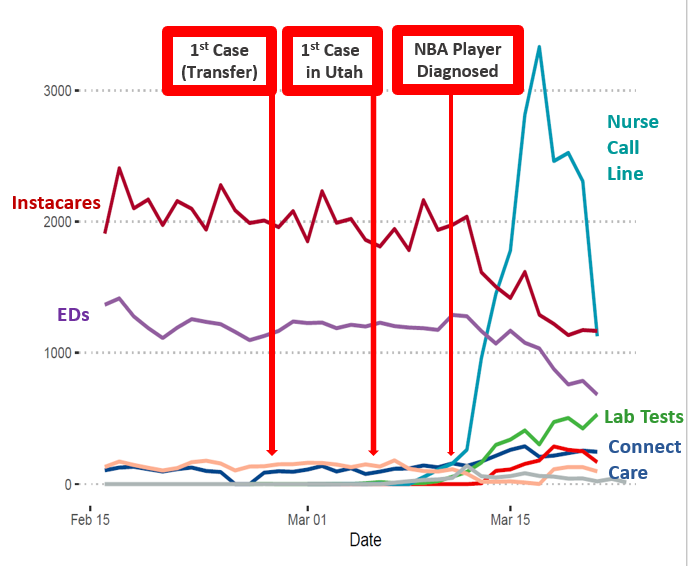
Dr. Todd J. Vento – Medical Director for the Infectious Diseases Telehealth Service at Intermountain Healthcare
Intermountain is leveraging its longstanding telehealth expertise to respond to the COVID-19 pandemic and increased requests for virtual communications and triaging.
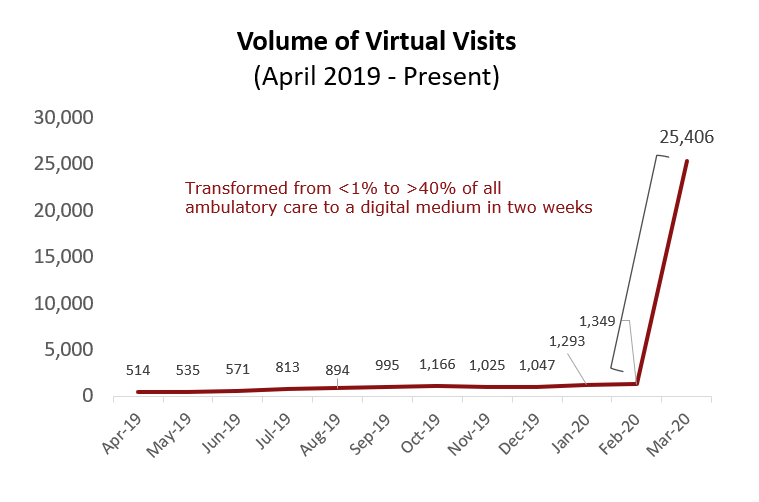
Dr. Lawrence “Rusty” Hofmann, Medical Director of Digital Health at Stanford Health
In two weeks Stanford Health Care scaled video visits from less than 1% to more than 40% of all ambulatory visits, screening patients with URI symptoms, reducing unnecessary exposure, minimizing community spread and maximizing medical resources.
Stanford also strongly encourages participation in their National Daily Health Survey — which will learn and predict which geographical areas will be most impacted by coronavirus based on patient-reported data.
Emergency Management Assistance Compact (EMAC) for Telehealth
Emergency Management Assistance Compact (EMAC) for Telehealth
The National Emergency Management Association released guidance on using the Emergency Management Assistance Compact (EMAC) to facilitate telehealth across state lines. EMAC is a national disaster-relief compact ratified by all 50 states, the District of Columbia, Puerto Rico and all U.S. territories.
On April 1, The National Emergency Management Association sent all 50 state governors a telehealth template for a gubernatorial executive order, which will create more consistency across state lines in implementing telehealth services and solving interstate licensure issues during the COVID-19 emergency. The Alliance for Connected Care and the National Governor’s Association called on Governors to use their authority under EMAC in March. Through this executive order, states can enable private sector telehealth in their state to assist health care workers.
If adopted, the template directs governors to waive interstate licensure for the duration of the emergency period, so that any health provider licensed, registered, or certified in good standing in another U.S. jurisdiction may deliver services in their state, including through telehealth, as long as those services are within the provider’s authorized scope of practice. This solves the existing telehealth barrier of interstate license permits and certificate reciprocity between states using existing EMAC state law. In the executive order template, governors can also mandate that no municipality, county or any other agency of the State can enact or enforce any order, rule, regulation, ordinance or resolution that would contradict the provisions included in the executive order.
EMAC Best Practices for Telehealth
 Loading...
Loading...
CMS COVID-19 Interim Final Rule Summary and Code Chart
As part of its announcement on “Sweeping Regulatory Changes to Help U.S. Healthcare System Address COVID-19 Patient Surge the Administration included significant additional Medicare telehealth changes, including:
- CMS will now pay for more than 80 additional services when furnished via telehealth. These include emergency department visits, initial nursing facility and discharge visits, and home visits, which must be provided by a clinician that is allowed to provide telehealth.
- CMS finalized CPT codes 98966-98968 and CPT codes 99441-99443 for prolonged, audio-only communication between the practitioner and the patient.
- Virtual Check-In services, or brief check-ins between a patient and their doctor by audio or video device, could previously only be offered to patients that had an established relationship with their doctor. Now, doctors can provide these services to both new and established patients.
- Clinicians can provide remote patient monitoring services for patients, no matter if it is for the COVID-19 disease or a chronic condition. For example, remote patient monitoring can be used to monitor a patient’s oxygen saturation levels using pulse oximetry.
- CMS is allowing telehealth to fulfill many face-to-face visit requirements for clinicians to see their patients in inpatient rehabilitation facilities, hospice and home health. During the pandemic, individuals can use commonly available interactive apps with audio and video capabilities to visit with their clinician.
- Home Health Agencies can provide more services to beneficiaries using telehealth, so long as it is part of the patient’s plan of care and does not replace needed in-person visits as ordered on the plan of care.
- Hospice providers can also provide services to a Medicare patient receiving routine home care through telehealth, if it is feasible and appropriate to do so.
- If a physician determines that a Medicare beneficiary should not leave home because of a medical contraindication or due to suspected or confirmed COVID-19, and the beneficiary needs skilled services, he or she will be considered homebound and qualify for the Medicare Home Health Benefit. As a result, the beneficiary can receive services at home.
- CMS is allowing for physician supervision requirements to be provided virtually, using real-time audio/video technology. Other changes to supervision requirements as well.
- Licensed clinical social worker services, clinical psychologist services, physical therapy services, occupational therapist services, and speech language pathology services can be paid for as Medicare telehealth services.
Please find a more detailed summary and a chart of the newly covered services below.
*Note that this interim final rule does not reflect several of the statutory changes achieved for telehealth in the “Coronavirus Aid, Relief, and Economic Security Act,” which was signed into law on March 27. That law altered preexisting relationship requirements for telehealth in Medicare and altered the telehealth payment structure for Federally Qualified Health Centers — among other changes.
For information on the COVID-19 waivers and guidance, and the Interim Final Rule, please go to CMS COVID-19 flexibilities webpage: https://www.cms.gov/about-cms/emergency-preparedness-response-operations/current-emergencies/coronavirus-waivers.
 Loading...
Loading...
 Loading...
Loading...