Medicaid Guidance Documents During the COVID-19 Pandemic
Medicaid Guidance from the Centers for Medicare and Medicaid Services
For purposes of Medicaid, telemedicine seeks to improve a patient’s health by permitting two-way, real-time interactive communication between the patient, and the physician or practitioner at the distant site. This electronic communication means the use of interactive telecommunications equipment that includes, at a minimum, audio and video equipment.
Reimbursement for Medicaid covered services, including those with telemedicine applications, must satisfy federal requirements of efficiency, economy, and quality of care. States are encouraged to use the flexibility inherent in federal law to create innovative payment methodologies for services that incorporate telemedicine technology. For example, states may reimburse the physician or other licensed practitioner at the distant site and reimburse a facility fee to the originating site. States can also reimburse any additional costs such as technical support, transmission charges, and equipment. These add-on costs can be incorporated into the fee-for-service rates or separately reimbursed as an administrative cost by the state. If they are separately billed and reimbursed, the costs must be linked to a covered Medicaid service.
CMS continues to issue and update guidance for states seeking to expand telehealth for Medicaid in response to the COVID-19 public health emergency. It is important to note that this guidance does not create new policy, but reminds states about the flexibility that exists in the Medicaid program. While Medicaid is jointly funded by federal and state governments, states have flexibility in designing and administering their program. This flexibility has created a great deal of variation in telehealth laws and reimbursement policies.
Recent federal guidance on teleheath in Medicaid:
- FAQs for State Medicaid Agencies and CHIP Agencies – Updated May 5
- Telehealth Toolkit to Accelerate State Use of Telehealth in Medicaid and CHIP – Updated December 6
- April 2 Rural Health Medicaid Telehealth Flexibilities and Medicaid SUD Treatment via Telehealth Guidance
- March 17 Medicaid Telehealth Guidance
- General Medicaid Telehealth Guidance
Please note that the Alliance has several pages devoted to state policy that may be relevant to Medicaid, including:
State Medicaid Telehealth Waivers & Temporary Flexibilities During COVID-19
The Alliance compiled a 50-state chart of Medicaid telehealth waivers and temporary flexibilities during COVID-19. For more information on Medicaid during the COVID-19 PHE please see our Medicaid Guidance Documents During the COVID-19 Pandemic.
 Loading...
Loading...
The Coronavirus Pandemic and the Transformation of Telehealth
The Coronavirus Pandemic and the Transformation of Telehealth
Find on our: COVID-19 Telemedicine Research & Reports
According to FAIR Health data, telehealth claim lines increased 4,347% nationally from March 2019 to March 2020 – with the most pronounced increase in the Northeast at more than 15,500% – further indicating the effects of the COVID-19 pandemic. In addition, most of the increase was found in mental health.
 Loading...
Loading...
 Loading...
Loading...
COVID-19 Telehealth Polling
The Alliance for Connected Care has compiled polls on patient and provider adoption, acceptance, and satisfaction with telehealth during the COVID-19 public health emergency. This chart has also been added to our Studies & Reports page. The Alliance will continue to update the chart with telehealth polls as they are published.
COVID-19 Telehealth Polls – Patient and Provider Adoption, Acceptance, and Satisfaction
| Study Group | Telehealth Polls: Summary | Date of Publication | Link to survey |
|---|---|---|---|
| Seniors | A poll from the University of Michigan’s Institute for Healthcare Policy and Innovation of more than 2,000 adults aged 50 to 80 finds an increase in telehealth visits from 4% as of May 2019 to 26% between March and June 2020. Other significant findings include: • They feel very or somewhat comfortable with video conferencing technologies: 64%, up from 53% in 2019 • At least one of their health providers offer telehealth visits: 62%, up from 14% • They are interested in using telehealth to connect with a provider they had seen before: 72%, up from 58% • They are interested in using telehealth for a one-time follow-up appointment after a procedure or operation: 63%, up from 55% • They have concerns about privacy during a telehealth visit: 24%, down from 49% • They are concerned they would have difficulty seeing or hearing the provider during a video visit: 25%, down from 39% | August, 2020 | Additional information can be found here. |
| A survey of more than 1,000 Medicare eligible consumers aged 64 and older conducted from July 17 to July 20 finds seniors are embracing telehealth and digital technologies. Telemedicine usage jumped 340% among Medicare-eligible seniors since the start of the COVID-19 pandemic and nearly one-third of consumers age 64 and older say they monitor their health using a wearable. Prior to COVID-19 only 1 in 10 used telemedicine. During COVID-19, 44% have used telemedicine and 43% say they intend on using it after, according to the survey. | August, 2020 | Additional information can be found here. | |
| A new poll of more than 1,000 seniors found 52% are comfortable using telehealth to receive health care. Of those who have used telehealth during the coronavirus, 91% reported a favorable experience, and 78% are likely to complete a medical appointment via telehealth again in the future. | May, 2020 | Additional information can be found here. | |
| Findings from the latest KFF Health Tracking Poll finds that the majority of older adults have an internet connection and communicate via smartphone, tablet, or computer to talk with friends. However, while 68% of adults 65 and older said they have a computer, smartphone, or tablet with internet access at home, only 11% said they have used the device to communicate with a health care provider in the past two weeks. KFF indicates that this number will likely rise as stay-at-home orders are extended. | April, 2020 | Additional information can be found here. |
|
| Adults | Change Healthcare and Harris Poll conducted a survey of more 2,000 Americans to better understand the consumer experience of finding, accessing, and paying for healthcare today. The vast majority of consumers agree that COVID-19 will fundamentally change how we receive healthcare in American, with 80% saying that COVID-19 has made telehealth an indispensable part of the healthcare system. • 3 in 4 consumers believe telehealth is the future of telemedicine. The vast majority of consumers agree that COVID-19 will fundamentally change how we receive healthcare in American, with 80% saying that COVID-19 has made telehealth an indispensable part of the healthcare system. • 65% plan to use telehealth more often than they did before the pandemic. 1 in 4 used telehealth for the first time due to COVID-19, while 16% have used it more often. 79% who used telehealth for the first time during the pandemic said they plan to use telehealth more in the future. | July, 2020 | The full report can be downloaded here. |
| A March survey found that 59% of the 500 U.S. consumers surveyed said they are more likely to use telehealth services now than previously, and 36% said they would switch their physician in order to have access to virtual care. | March, 2020 | Additional information can be found here. | |
| A survey of 2,000 adults across the U.S. on perceptions of telehealth during COVID-19 found that more than 95% of respondents who had used telehealth said they already have or would consider scheduling another telehealth appointment in the future. The most cited advantages to telehealth were quicker and greater access to care and avoiding overcrowded wait rooms. | March, 2020 | Additional information can be found here. | |
| Providers | A survey of more than 1,300 physicians found that more than 90% are treating some or all of their patients via telehealth. Additionally, roughly 60% of physicians currently using telemedicine tools during the public health emergency said they plan to use telemedicine more often than they were pre-COVID. | April, 2020 | Additional information can be found here. |
| A survey of more than 800 physicians found that close to half (48%) are treating patients via telemedicine, up from 18% in 2018. | April, 2020 | Additional information can be found here. | |
| A survey found that all 20 accountable care organizations (ACOs) surveyed are implementing telemedicine solutions, with 16% relying on AI and automation to identify and reach high-risk patients. | April, 2020 | Additional information can be found here. | |
| A survey of more than 600 healthcare providers found that 41% were using telemedicine technology, up from 22 percent in a 2018 survey. In addition, roughly 28% of the practices surveyed offered telehealth-only visits. | March, 2020 | Additional information can be found here. | |
| Other | The latest Modern Healthcare CEO Survey finds that health system CEOs see a wave of innovation in telehealth over the next year. In addition, 92.9% of CEOs cited telehealth as a technology with the most potential to support response to the COVID-19 pandemic. | May, 2020 | Additional information can be found here. |
April CMS COVID-19 Interim Final Rule Summary
On April 30, the Centers for Medicare and Medicaid Services has released another Interim Final Rule, implementing significant additional changes for telehealth. Specifically, this Interim Final Rule includes more changes created using the statutory authority of Coronavirus Aid, Relief, and Economic Security Act (CARES Act). These changes come from both new 1135 waivers and the interim final rule – both summarized in the below document.
- CMS expanded the ability to practice telehealth services to all providers eligible to bill Medicare. This should fix concerns about physical therapists, occupational therapists, speech language pathologists, and others not being on the distant site provider list.
- CMS increased Medicare payment rates for the previously-created audio-only E&M codes, but has not broadly allowed audio to be used for all telehealth delivery. There are focused expansions of audio-only listed here and below in the summary.
- CMS gave itself the authority to make future changes to the telehealth services list through sub-regulatory guidance.
- Rural Health Clinics and Federally Qualified Health Centers may bill Medicare for telehealth as per the CARES Act.
CMS Resources:
- CMS Press release
- Regulation: Medicare and Medicaid IFC: Additional Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency (CMS-5531 IFC) (PDF)
- CMS 1135 Blanket Waivers
Summary:
 Loading...
Loading...
Telehealth Guidance Documents for Employee Benefit Plans
The Departments of Labor (DOL), Health and Human Services (HHS), and the Treasury prepared FAQs regarding implementation of the Families First Coronavirus Response Act (FFCRA) and the CARES Act. Section 6001 of the FFCRA requires group health plans and health insurance issuers offering group or individual health insurance coverage to provide benefits for certain items and services related to diagnostic testing for the detection of or the diagnosis of COVID-19. Under the FFCRA, plans and issuers must provide this coverage without imposing any cost-sharing requirements (including deductibles, copayments and coinsurance) or prior authorization or other medical management requirements.
Further, DOL issued additional FAQs designed to help employee benefit plan participants and beneficiaries, as well as plan sponsors, and employers, impacted by the COVID-19 outbreak understand their rights and responsibilities under Title I of the Employee Retirement Income Security Act of 1974 (ERISA). This guidance – in reference to the above FAQs – permits plans to:
- Add telehealth and other remote health services, in addition to services related to diagnosis and treatment of COVID-19, mid-year and without providing 60 days’ advance notice as required under federal law.
- COVID-19 FAQs for Participants and Beneficiaries
Alliance Calls for More Broadband Funding
The Alliance for Connected Care endorsed H.R.6474 – the Healthcare Broadband Expansion During COVID-19 Act. This legislation would significantly expand funding for the Federal Communications Commission to support healthcare broadband deployment in response to COVID–19.
 Loading...
Loading...
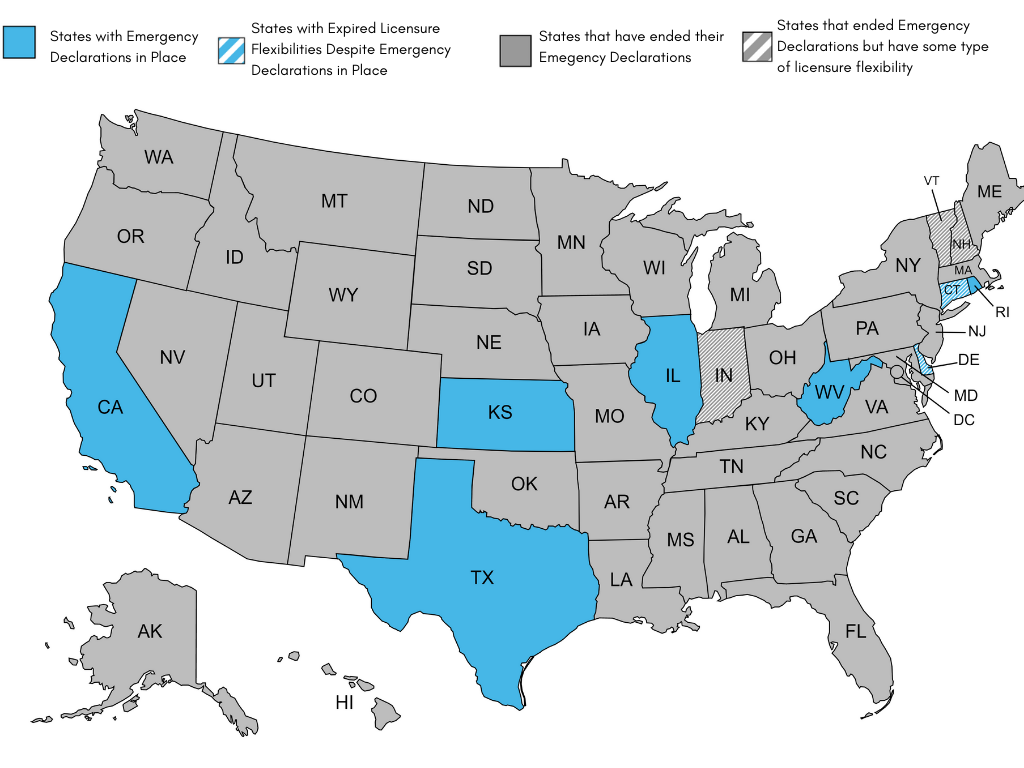
COVID-19 State Telehealth and Licensure Expansion Dashboard
State Expansion of Telehealth and Licensing Waivers
During the public health emergency, all 50 states and the District of Columbia used emergency authority to waive some aspect(s) of state licensure requirements to facilitate patients getting care. This has provided an unprecedented opportunity for patients, providers, and policymakers to explore the impact of cross-state care. This has benefited the delivery of health care in many ways, but perhaps most notably, it has opened up many new avenues for patient choice and access to care.
As states begin to lift their COVID-19 emergency waivers or let them expire, many of the telehealth and licensure flexibilities enacted at the start of the pandemic to ensure continuity and access to care for patients are also expiring. As such, the Alliance has created a chart outlining which states have lifted their COVID-19 emergency waivers, and how this has impacted telehealth and licensing flexibilities in each state. This document is no longer updating this document after its last updated date given most states have or plan to terminate their emergency declarations. It was last updated on December 16, 2022.
As of December 16, 2022:
- 42 states and D.C. have ended their emergency declarations: AL, AK, AZ, AR, CO, DC, FL, GA, HI, ID, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, SC, SD, TN, UT, VT, VA, WA, WI, WY.
- IN, NH, and VT, however, have licensure flexibilities still in place.
- IN will extend out-of-state health care registry through the duration of the federal PHE.
- NH SB 277 extends the expiration for temporary and emergency licenses for health care workers, if they were obtained on or before Jan. 31, 2022, through June 30, 2023.
- VT passed legislation that extends pandemic-era license waivers through June 30, 2023.
- IN, NH, and VT, however, have licensure flexibilities still in place.
- 8 states continue to have emergency declarations in place: CA, CT, DE, IL, KS, RI, TX, WV.
- Of these 8 states, 6 states still have licensure flexibilities in place. Licensure flexibilities have expired in CT and DE, despite emergency declarations still in place.
- CA is going through a phased rollback of COVID-era waivers, however waivers on telehealth and licensure are still in place.
- In total, 9 states still have licensure flexibilities in place.
Note: The Alliance is no longer updating this document after its last updated date given most states have or plan to terminate their emergency declarations. It was last updated on December 16, 2022.
Figure 1. Map highlighting status of COVID-era telehealth and licensure waivers state-by-state. Last updated December 16, 2022.
 Loading...
Loading...
 Loading...
Loading...
CMS Approved 1135 Waivers
Under a disaster or emergency declaration and a public health emergency, the HHS Secretary is authorized to take additional actions to provide programmatic flexibility in Medicare, Medicaid and the Children’s Health Insurance Program through section 1135 waivers. The Secretary may issue blanket waivers, or may approve state specific waivers. Waivers end no later than the termination of the emergency period. CMS released a checklist for states to aid in the development of 1135 waivers requests.
COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers
Several states requested flexibility to incent greater use of telehealth through Medicaid Section 1135 Waivers during the pandemic. For example:
- IL, LA, NC, and WA requested CMS to allow providers to use non-HIPAA compliant telehealth modes from platforms like Facetime, WhatsApp, and Skype to facilitate visits.
- CA requested flexibility to make it easier for providers to care for people in their own homes by allowing telehealth and virtual/telephonic communications for covered State plan benefits, a Waiver of face-to-face encounters for FQHCs and Rural Health Clinics, and Reimbursement of virtual communication and e-consults for certain providers.
- MD requested flexibility so that Medicaid and Managed care enrollees could use telephones to receive care if they did not have an appropriate device.
- SD requested flexibility to allow Medicaid to pay for the same telehealth services that Medicare has been granted authority to pay for, including services furnished while a patient is at home.
As of April 2020, CMS had approved 53 state waivers. A full list of the approved 1135 waivers (last updated April 23, 2020) can be found here.
COVID-19 Resources and Guidance from Alliance Members and Partners
The Alliance is closely monitoring developments regarding COVID-19 and will continue to update this page. Please send resources to cadamec@connectwithcare.org for inclusion on this page.
The Alliance has compiled a resource detailing state-by-state expansion of telehealth and licensing waivers during the COVID-19 pandemic. during the COVID-19 pandemic. The Alliance also has a list of federal agency telehealth guidance documents. These pages are updated regularly.
Recent Alliance webinars on COVID-19:
- Video: Using Telehealth to Address the Coronavirus Public Health Emergency. During this event, telehealth leaders from Stanford, Intermountain, and Medstar Health spoke to what their systems are doing and how they delivering dramatically more care via telehealth during the COVID-19 pandemic.
- Video: Lunch and Learn – Telehealth Policy Developments COVID-19 and Beyond. During this event, the Alliance reviewed the multitude of legislative and regulatory changes for telehealth and remote patient monitoring over the past month and discussed new opportunities beyond COVID-19.
External Telehealth Resources:
American Academy of Family Physicians telehealth resources page featuring tools for practices to build telehealth capacity. Additionally, the Financial Relief for Family Physicians page breaks down available financial relief programs, plus offers a related cost calculator, Medicare Administrative Contact list and application instructions.
American Association of Nurse Practitioners (AANP)has a tracking page with federal and state policy resources, including an Emergency State Licensure page tracking state orders to consider emergency licensing for the nurse workforce to meet increased demands resulting from COVID-19.
American Academy of PAs (AAPA) website includes a map showing the states that have taken action to suspend or waive certain practice requirements for PAs in response to the COVID-19 pandemic.
American Medical Association (AMA)has a page with special coding advice during the COVID-19 public health emergency.
American Speech-Language-Hearing Association telehealth resources page includes State-by-State Tracking of Laws and Regulations for Telepractice and Temporary Practice and COVID-19 Commercial Insurance Telepractice Policy Tracking.
American Urological Association page on telehealth and urology during COVID-19.
Association for Behavioral Health and Wellness lists COVID-19 Behavioral Health Resources
The California Health Care Foundation has resources page on telehealth delivery, with a specific emphasis on California Telehealth Policy changes .
Center for Connected Health Policy includes a CCHP COVID-19 Related State Actions to Date – States waiving licensure requirements/renewals and states waiving in-state licensure requirements for telehealth and CCHP COVID-19 Telehealth Coverage Policies.
National Alliance on Mental Illness (NAMI) NAMI released a NAMI COVID-19 Resource and Information Guide to answer frequently asked questions regarding the intersection between Coronavirus, or COVID-19, and people affected by mental illness, their caregivers and loved ones.
National Association for Home Care & Hospice Coronavirus resource page for home health and hospice community includes Info for Patients & Family Caregivers
National Council for Behavioral Health site includes resources for behavioral health organizations including economic assistance for small businesses, training and technical assistance resources, and a COVID-19 Guidance for Behavioral Health Residential Facilities.
National Consortium of Telehealth Resource Centers has a COVID-19 Telehealth Toolkit provides an overview of telehealth policy, how telehealth can be used in response to COVID-19 and additional resources. Video: Telehealth Best Practices for Providers (Providers) Video: What to Expect from a Telehealth Visit (Patients)
Alliance Comments on the COVID-19 Interim Final Rule
On April 14, the Alliance for Connected Care wrote the Centers for Medicare and Medicaid Services in response to the COVID-19 Interim Final Rule. The Alliance emphasized the importance of the many changes that were made, and requested several next steps for CMS, leveraging new authorities that is has received under the Coronavirus Aid, Relief, and Economic Security Act. Alliance priorities included:
- Expanded flexibility for audio-only telehealth to a broader set of services
- Technical fixes to ensure telehealth can be delivered by more clinicians, including changes to distant site provider rules.
- Additional flexibility for E-Visits and Virtual Check-ins
- Ensure robust data collection during this time period so that the nation may learn from its experience with telehealth and remote patient monitoring
- Expand efforts to educate Medicare beneficiaries about utilizing telehealth.
 Loading...
Loading...